Japan In-vitro Diagnostics Market Size

| Study Period | 2019 - 2029 |
| Base Year For Estimation | 2023 |
| Forecast Data Period | 2024 - 2029 |
| Historical Data Period | 2019 - 2022 |
| CAGR | 4.50 % |
Major Players
*Disclaimer: Major Players sorted in no particular order |
Japan In-vitro Diagnostics Market Analysis
The field of in-vitro diagnostics has witnessed several changes and additions to its gamut of offerings in the recent past. There has been a paradigm shift from traditional diagnostics to a new generation diagnostics that work on the gene level. This was possible due to the inclusion of advanced technology, such as genetic testing, molecular diagnostics, Polymerase Chain Reaction (PCR), and Next-Generation Sequencing (NGS). Fast turnaround, reliability, user-friendliness, and predictability of predisposed diseases are few significant qualities, which helped these technologies attain their share in major offerings of diagnosis providers around the world and especially Japan. Increasing demand from the educated public for more information about their predisposition for serious diseases, and how these potential illnesses can be detected at an early stage, is driving the market for Japan in-vitro diagnostics. Furthermore, a growing burden of chronic and infectious diseases, increasing use of point of care (POC) diagnostics, and surging number of private hospitals and independent testing laboratories are some of the other factors contributing to the growth of the market. However, lack of proper reimbursement system in Japan, stringent regulations and limited budget of hospitals and laboratories in developing economies are also restraining the japan in-vitro diagnostics market.
Japan In-vitro Diagnostics Market Trends
This section covers the major market trends shaping the Japan In-vitro Diagnostics Market according to our research experts:
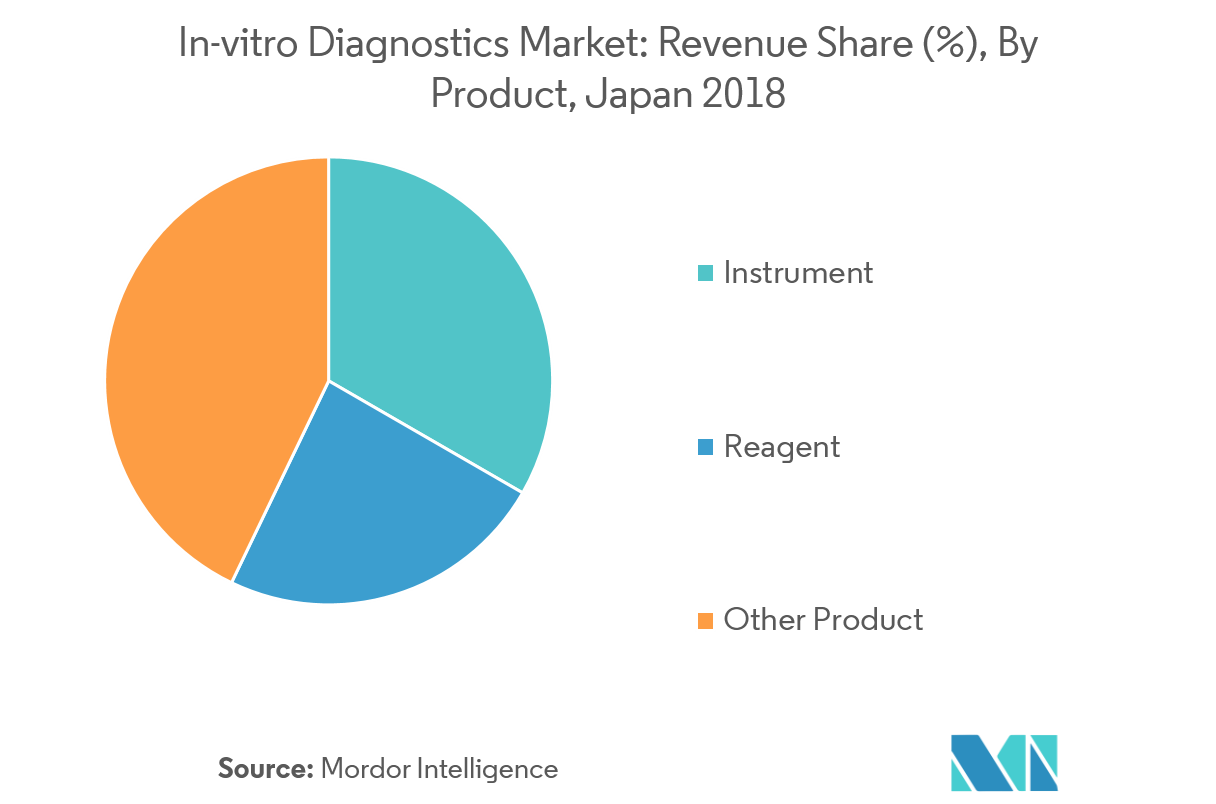
Instruments segment dominates the Japan In-vitro Diagnostics Market
The population of Japan is aging rapidly. The health life expectancy has shown a dramatic increase in recent years. This trend is expected to shift the focus of the medical device industry from treatment to prevention. The IVD instruments are expected to play a major role in this shift. IVD instruments, such as glucose meters, chemical analyzers, clinical chemistry analyzers, hematology analyzers, urine analyzers, and immunochemistry analyzers are expected to find increased applications, with people starting to prefer prevention over treatment. Companies are increasing their R&D expenditures, and are continuously bringing integrated and closed systems to the market, in order to retain their market share. Moreover, outsourcing in instrumentation is increasing gradually instead of in-house instrumentation, owing to increased efficiency, speed, and low operating costs realized, due to the economics of scale. The aforementioned factors are expected to increase the market, during the forecast period.
Japan In-vitro Diagnostics Industry Overview
The market comprises of major market players and these players are moreover focusing on research and development in order to form a stable and safe formulation. The market has been noticing technological developments on a large scale for the past couple of years. For instance, in July 2017 Sysmex Corporation and bioMérieux announced that they had agreed to transfer all of Sysmex holdings in Sysmex bioMérieux Co., Ltd. (Tokyo, Japan) to bioMérieux. Key players in Japan In-vitro Diagnostics market are Biomérieux, Abbott Laboratories, Bayer AG, Bio-Rad Laboratories, Inc., Danaher Corporation, Johnson & Johnson, Nippon Becton Dickinson Company, Roche Diagnostics and Siemens amongst others.
Japan In-vitro Diagnostics Market Leaders
-
Abbott
-
Becton Dickinson & Company
-
Biomerieux
-
Bio-rad Laboratories Inc.
-
Qiagen
*Disclaimer: Major Players sorted in no particular order
Japan In-vitro Diagnostics Market Report - Table of Contents
1. INTRODUCTION
- 1.1 Study Deliverables
- 1.2 Study Assumptions
- 1.3 Scope of the Study
2. RESEARCH METHODOLOGY
3. EXECUTIVE SUMMARY
4. MARKET DYNAMICS
- 4.1 Market Overview
-
4.2 Market Drivers
- 4.2.1 High Prevalence of Chronic Diseases
- 4.2.2 Increasing Use of Point-of-Care (POC) Diagnostics Spurring the IVD Market
- 4.2.3 Advanced Technologies Fueling the Japan IVD Market
- 4.2.4 Increasing Awareness and Acceptance of Personalized
- 4.2.4.1 Medicine and Companion Diagnostics
-
4.3 Market Restraints
- 4.3.1 Lack of Proper Reimbursement
- 4.3.2 Stringent Regulatory Framework
-
4.4 Porter's Five Force Analysis
- 4.4.1 Threat of New Entrants
- 4.4.2 Bargaining Power of Buyers/Consumers
- 4.4.3 Bargaining Power of Suppliers
- 4.4.4 Threat of Substitute Products
- 4.4.5 Intensity of Competitive Rivalry
5. MARKET SEGMENTATION
-
5.1 By Test Type
- 5.1.1 Clinical Chemistry
- 5.1.2 Molecular Diagnostics
- 5.1.3 Immuno Diagnostics
- 5.1.4 Haematology
- 5.1.5 Other Types
-
5.2 By Product
- 5.2.1 Instrument
- 5.2.2 Reagent
- 5.2.3 Others
-
5.3 By Usability
- 5.3.1 Disposable IVD Devices
- 5.3.2 Reusable IVD Devices
-
5.4 By Application
- 5.4.1 Infectious Disease
- 5.4.2 Diabetes
- 5.4.3 Cancer/Oncology
- 5.4.4 Cardiology
- 5.4.5 Autoimmune Disease
- 5.4.6 Nephrology
- 5.4.7 Other Applications
-
5.5 By End Users
- 5.5.1 Diagnostic Laboratories
- 5.5.2 Hospitals & Clinics
- 5.5.3 Other End Users
6. COMPETITIVE LANDSCAPE
-
6.1 Company Profiles
- 6.1.1 Abbott Laboratories
- 6.1.2 F Hoffmann La Roche
- 6.1.3 Becton Dickinson & Company
- 6.1.4 Danaher Corporation
- 6.1.5 Biomerieux
- 6.1.6 Bio-rad Laboratories Inc.
- 6.1.7 Thermofisher Scientific Inc.
- 6.1.8 Qiagen
- 6.1.9 Siemens Healthineers
- 6.1.10 Sysmex
- *List Not Exhaustive
7. MARKET OPPORTUNITIES AND FUTURE TRENDS
** Subject To AvailablityJapan In-vitro Diagnostics Industry Segmentation
As per the scope of this report, in vitro diagnostics involves medical devices and consumables that are utilized to perform in vitro tests on various biological samples. They are used for the diagnosis of various medical conditions, such as chronic diseases.
| By Test Type | Clinical Chemistry |
| Molecular Diagnostics | |
| Immuno Diagnostics | |
| Haematology | |
| Other Types | |
| By Product | Instrument |
| Reagent | |
| Others | |
| By Usability | Disposable IVD Devices |
| Reusable IVD Devices | |
| By Application | Infectious Disease |
| Diabetes | |
| Cancer/Oncology | |
| Cardiology | |
| Autoimmune Disease | |
| Nephrology | |
| Other Applications | |
| By End Users | Diagnostic Laboratories |
| Hospitals & Clinics | |
| Other End Users |
Japan In-vitro Diagnostics Market Research FAQs
What is the current Japan In-vitro Diagnostics Market size?
The Japan In-vitro Diagnostics Market is projected to register a CAGR of 4.5% during the forecast period (2024-2029)
Who are the key players in Japan In-vitro Diagnostics Market?
Abbott, Becton Dickinson & Company, Biomerieux, Bio-rad Laboratories Inc. and Qiagen are the major companies operating in the Japan In-vitro Diagnostics Market.
What years does this Japan In-vitro Diagnostics Market cover?
The report covers the Japan In-vitro Diagnostics Market historical market size for years: 2019, 2020, 2021, 2022 and 2023. The report also forecasts the Japan In-vitro Diagnostics Market size for years: 2024, 2025, 2026, 2027, 2028 and 2029.
Safety IO Modules Industry Report
Statistics for the 2024 Safety IO Modules market share, size and revenue growth rate, created by Mordor Intelligence™ Industry Reports. Safety IO Modules analysis includes a market forecast outlook 2029 and historical overview. Get a sample of this industry analysis as a free report PDF download.



